Thesis | Proteasomal inhibition and Parkinson’s disease modelling
Research field(s): Molecular neurosciences, medicinal chemistry, biopharmaceutics, drug design.
Thesis title: Investigation into the physicochemical and biological properties of the proteasomal specific inhibitor I (PSI).
Degree: PhD.
Supervisors: Prof. Peter Jenner, Prof. Robert Hider, Dr. Sarah Salvage, and Dr. Sukhvinder Bansal.
Where are you undertaking/did you undertake your research?
I undertook my PhD at King’s College London, one of the leading research universities based in the heart of London. The project was a result of collaboration between the Neurodegenerative Diseases Research Centre, the Institute of Pharmaceutical Sciences and Proximagen Ltd.
What is your thesis about?

Photomicrographs of tyrosine hydroxylase (TH)-positive dopaminergic neurons in the substantia nigra (SN) following PSI administration. (Image©NSRC Labs)
The thesis details a novel approach into the synthesis of the proteasomal specific inhibitor I (PSI), a synthetic neurotoxin which is used as a probing tool to model the pathological hallmarks of Parkinson’s disease in-vitro. and in-vivo. The investigations I carried in my PhD provide a detailed account on the physicochemical, pharmacokinetic and pharmacodynamic properties of the modelling tool. The overall aim is to gain a better understanding of PSI-mediated neurodegeneration and optimising the neurotoxin-based model of Parkinson’s disease as a reliable translational research tool to bridge the gap between the currently employed model systems of neurodegeneration and subsequent clinical evaluation of putative neuroprotective strategies. The lines of investigation presented in the thesis also provide an insight into the key pharmacological effects induced by PSI administration following in-vitro and in-vivo assessments of the underlying mechanisms of PSI-induced neurodegeneration.
How did you get into this research?
I Undertook three very successful industrial studentships in the summers of 2004, 2005 and 2006 in the fields of drug delivery/formulation, synthetic chemistry and neuropharmacology, which had a tremendous impact on my interest in pharmaceutical research. The latter project was particularly engaging and exciting because it involved testing novel neuroprotective agents in-vitro. My growing interest in the fields of molecular neurosciences, drug design and medicinal chemistry eventually culminated in pursuing a postgraduate degree on neurodegenerative disorders.
Why is it important?
The body of evidence accumulated on the clinical management of Parkinson’s disease (PD) seems to indicate that the currently prescribed medication only aims to provide sufficient palliative response and functional capacity improvement in

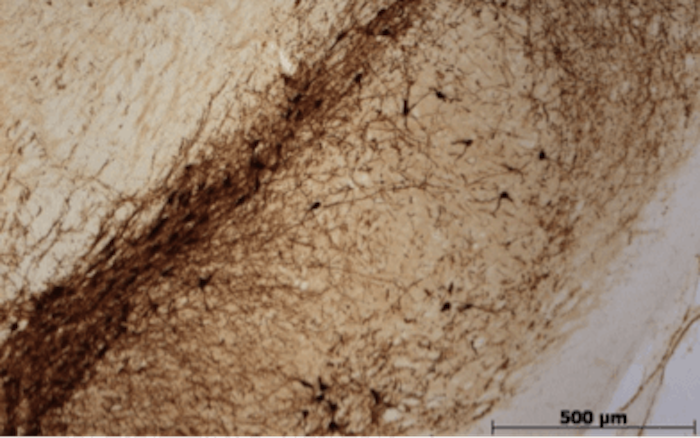
The formation of ubiquitin-positive Lewy body (LB)-like aggregates in the cytoplasm of TH-positive dopaminergic neurons in the SN following PSI administration. (Image©NSRC Labs)
the early stage of illness. However, the long-term prognosis is invariably accompanied by poorly managed complications and irreversible disease deterioration. This justifies the never-ending efforts to design neuroprotective agents which potentially halt or reverse the degenerative process.
Clinical trials of such neurorestorative strategies have routinely failed despite their unmatched efficacy in experimental models. This is primarily owed to the failure of such pre-clinical model systems to recapitulate the heterogeneity of the clinicopathological features of PD. One novel approach which has been recently shown to assimilate the complex progressive pattern of neurodegeneration involves the inhibition of the proteasome in the substantia nigra using the neurotoxin PSI. Despite the initial unprecedented success reported using this model, failure to reproduce the original findings and the poor understanding of underlying pharmacology raised doubts over the reliability of the model to test neuroprotective agents.
What is your contribution to your field of research?
The concerted effort conducted in the project emphasises the importance of the physicochemical and pharmacokinetic properties of the neurotoxin as core contributors to the poor reproducibility of its pharmacodynamics. The major contention put forward in the thesis also underscores the implications of structural limitations and drug design approaches to optimise its pharmacological effects and replication of the clinicopathological profile of Parkinson’s disease. It also reduces enormous gaps in the present state of knowledge by proposing new mechanisms to PSI-induced neurodegeneration which offers reasons for optimism considering the central role of proteasomal inhibition in PD modelling.
About the Author